Automated Assays with 3D Cell Models in ECM and Hydrogels
Introduction
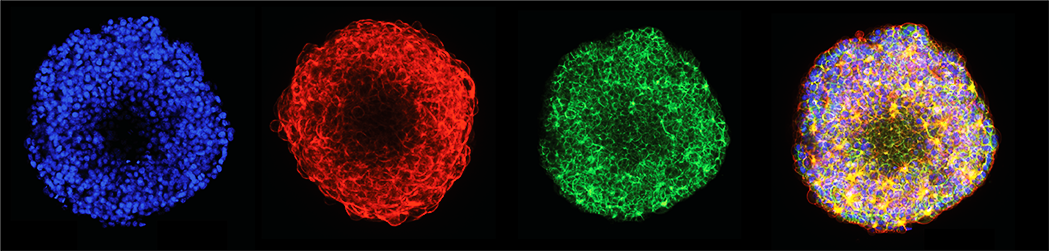
For decades cell biology principles, drug activities, cell responses, and tissue morphogenesis have been determined in 2D cell monolayer culture systems. Such bi-dimensional cell environment is unnatural and lacks most of the interactions occurring in the native 3D tissues. 3D in vitro cell culture systems have become increasingly popular and have great potential as tools for disease modeling and drug discoveryThis platform integrates 3D cell model formation, sample interrogation, fixation, staining, and high-content imaging in the flowchip; providing high-quality imaging data for phenotypic profiling. We have established protocols using either complex basement membrane matrix (e.g., Matrigel® and HS murine sarcoma ECM gel from Sigma-Aldrich) or engineered hydrogels. Although Matrigel is widely used, it has assay limitations like undefined composition, lot-to-lot variability, murine components, and temperature dependency. Recently developed synthetic alternatives can provide a chemically defined, xenogeneic-free environment that can be modified for desired outcomes and provide reproducible results. In this application note we demonstrate applications using ECMs and engineered hydrogels for automated image-based profiling 3D assays (Figure 1). We present the use of the Pu·MA System for three different 3D cell culture applications: 1. Formation of 3D cell models in ECM 2. Cell marker detection of 3D cell models in ECM 3. Profiling engineered alveolar organoids in hydrogel
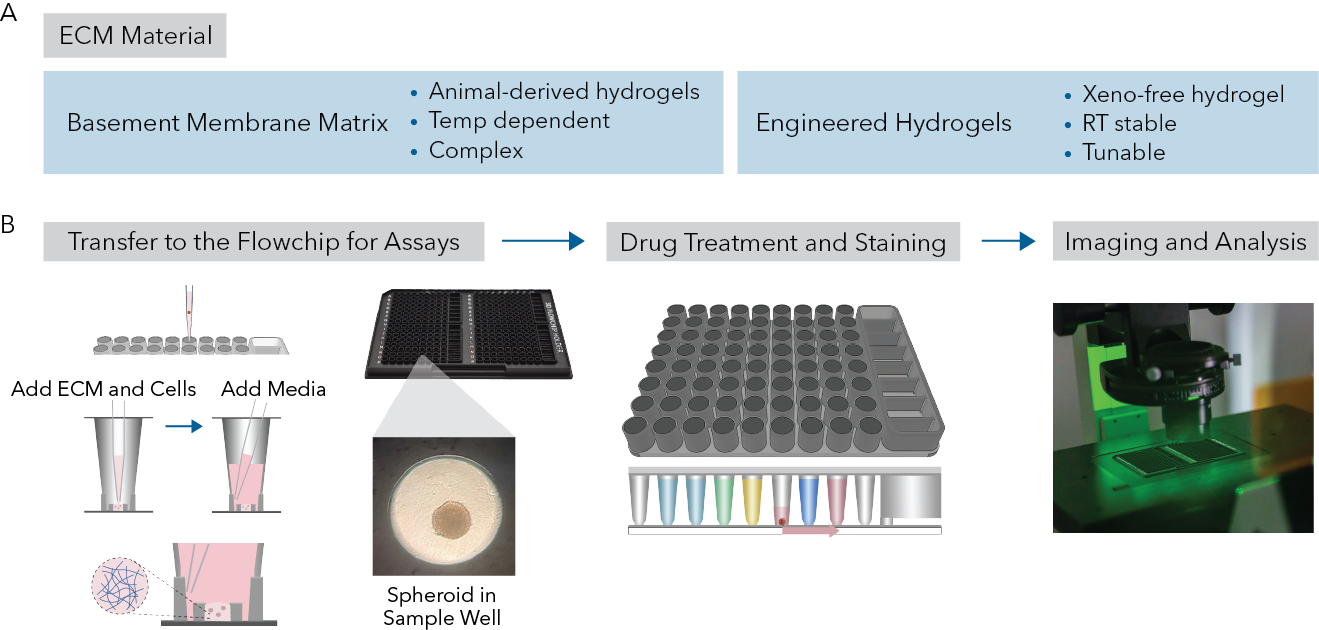
Fig 1. (A) Different types of ECMs used in this app note. (B) The workflow includes, creating minidomes with 3D cell model in ECM within the protected sample chamber of the sample well, flowchips loaded with assay reagents for automated assays using Pu·MA System, followed by imaging of 3D cell models within the flowchip.
