Single Spheroid Metabolomics
Sample preparation for metabolomics analysis always involves transferring samples to ice after compound or metabolite treatment. This affects the metabolic profile. To overcome this drawback, we can enable automated workflows using the Pu·MA System 3D.
You perform drug treatments during the course of an assay without disturbing the spheroid and subsequently add in the lysing solution at a specified time point to lyse the spheroid in situ before analysing the lysed samples by mass spec analysis.
The tabs below showcase this application of in situ lysis for metabolomics using the Pu·MA System.
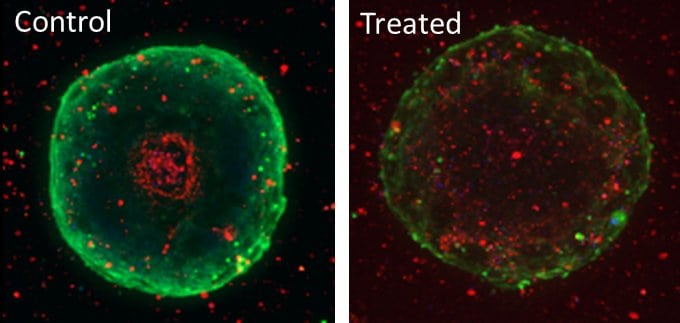
Use of Pu·MA System 3D for Single Spheroid Assays with Downstream Metabolomics
This application note demonstrates the use of the Pu·MA System for spheroid treatment with drugs followed by in situ lysis for preparing samples for downstream metabolomics analysis.

Automated Long-Term 3D Cell-Based Toxicity Studies Using a Flowchip System
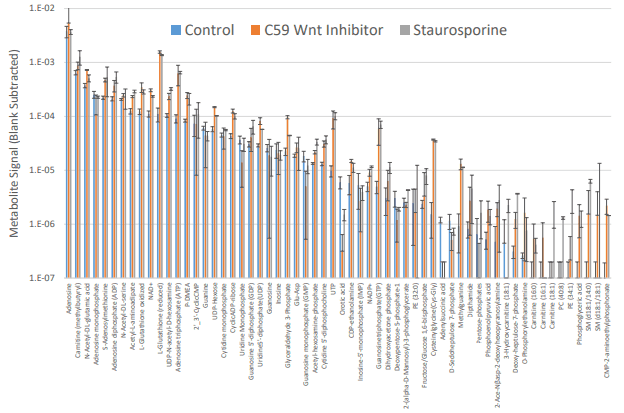
We presented data about how you can single spheroid drug treatment and dosing. The spheroid samples were lysed within the incubator and samples analyzed for metabolic changes.
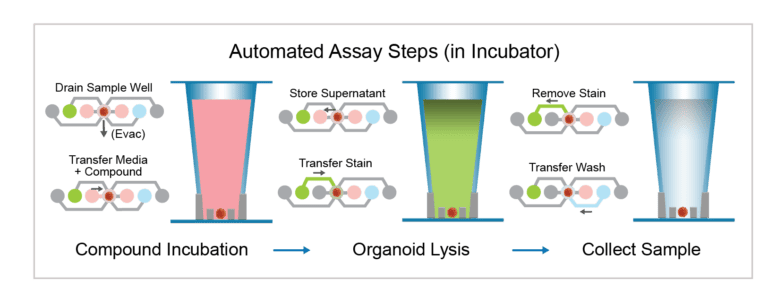
Metabolomics Prep Workflow
Organoids, media and reagents are loaded into the flowchips. The Pu·MA System performs the automated steps described below in the workflow.
Contact Us
Protein Fluidics, Inc.
875 Cowan Road, Suite B,
Burlingame, CA 94010
+1 650 529 5080
info@proteinfluidics.com
#pumasystem #flowchip #3dcellassay
Our Company
