
Automated Immunofluorescence Staining and Imaging of Breast Cancer Spheroids
Introduction
Biological systems are very complex and are not accurately reflected in conventional 2D monolayer cultures. The adoption of 3D cell models is growing in both academic research and drug discovery because they better represent human biology, physiological processes, and disease phenotype 1-4 . This adoption is reflected in increased journal publications and the development of commercial products that facilitate 3D cell-based assays 5 . Biomarkers are considered to be the cornerstones of preventive and personalized medicine. Biomarker research conducted in 3D models has higher clinical relevance than 2D. Immunofluorescence staining (IF) is a widely used method to identify and quantify specific biomarker expression and cellular localization. One of the challenges researchers face with IF staining using precious spheroids or organoids, is that it tends to be manual and tedious. Culture systems like hanging drop or ultra-low attachment are also difficult to handle for IF staining due to the high risk of spheroid loss or damage. There are further technical challenges of plate compatibility and locating spheroids during confocal microscopy, which is typically used for imaging of stained spheroids. Also, the commonly used approach of spheroid embedding, and sectioning is extremely cumbersome, time consuming and prone to errors. To overcome these challenges, we have developed and describe here a breakthrough workflow for automated IF staining of 3D spheroids using microfluidic-based Pu·MA System® 4,6,7. In this technote, we demonstrate automated IF staining of MCF7 breast cancer spheroids to detect the biomarkers E-cadherin (an epithelial cell-cell adhesion molecule), multiplexed with Phalloidin (for F-actin) and Hoechst (nuclei) labeling. The assay was performed with the spheroids suspended in 25% Matrigel and imaged within the flowchip using CellVoyager CQ1 High-Content Analysis System from Yokogawa Electric Corporation. The workflow presented here has high utility and value for studying the interplay between cadherin cell adhesion machinery and associated partners like F-actin, its role in epithelial-to-mesenchymal transition and metastasis, as well as modulation dynamics by pharmacological agents.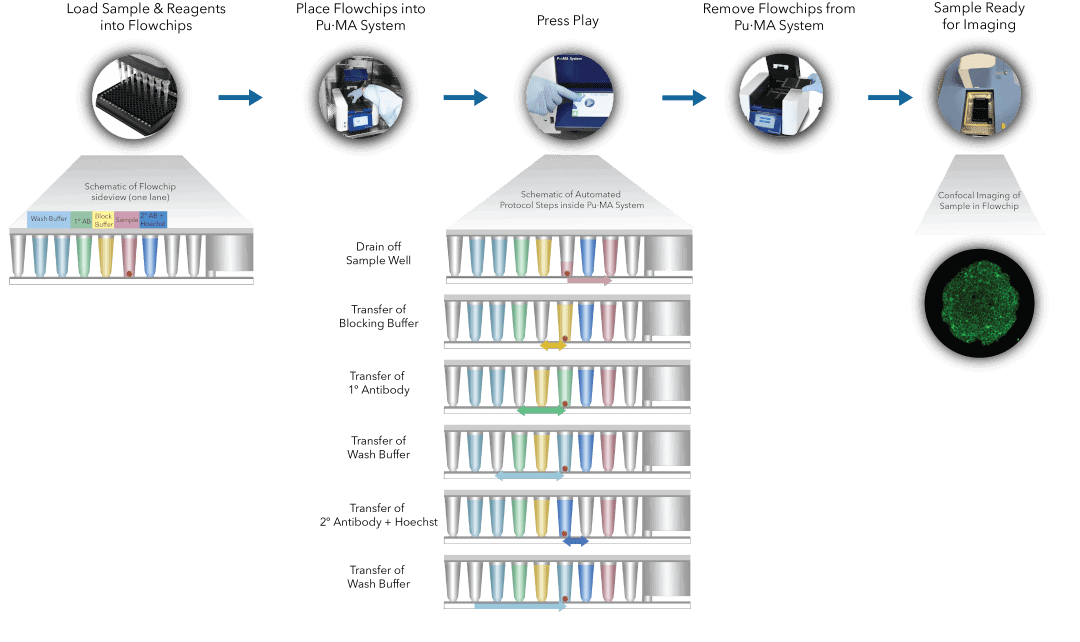
Fig 1. Pu·MA System IF staining workflow schematic representation of automated steps within the Pu·MA System followed by subsequent confocal imaging within flowchips
