Multi-parametric Human Cell Based Inflammation Assay for Cytokines and Cell Surface Antigens
Introduction
Inflammation is a complex event in which cells respond to various endogenous and exogenous stimuli. Factors such as tumor necrosis factor alpha (TNF-α), interleukin-1 beta (IL-1β), and interferon gamma (IFN-γ) activate signaling pathways leading to the expression of cell-surface antigens that facilitate binding of immune cells to blood vessels. The ability to monitor up-regulation of molecules, such as MCP-1, IL-8, IL-6, VCAM, and HLA-DR with endothelial cells provides an important physiological read-out for cell-based models of inflammation. We present results from a multiparametric primary human cell-based assay that uses immunoassays for secreted cytokines and fluorescence read-outs of cell surface markers to evaluate the effect of different mediators on inflammatory response. The combination of imaging and microfluidic-based assays provides an efficient multiparametric assay system that can be used to test the efficacy of anti-inflammatory compounds versus toxicity and also provide significant insight into the mechanism of action by selective inhibition of markers triggered by different signaling pathways.
Assay Background
Endothelial cells play a critical role in inflammation by responding to several endogenous and exogenous proinflammatory stimuli.1 Here we assessed differences in signaling pathways, adhesion molecules, and cytokines induced by proinflammatory factors (IFN-γ, TNF-α, and IL-1β) in human umbilical vein endothelial cells (HUVEC) using multi-parametric readouts.2 Expression of the inflammation markers on primary human umbilical vein endothelial cells (HUVEC) stimulated with inflammation cytokines (TNF-α, IFN-γ, and IL-1β) was quantified by measurement of total fluorescence intensity after staining with directly conjugated antibodies and low-volume ELISAs. The Pu·MA System was used to quantify MCP-1, IL-8, and IL-6 in cell supernatants. The ImageXpress® Nano Automated Imaging System was used to monitor cells surface expression of VCAM and HLADR. The PuMA System runs automated low-volume ELISAs using existing antibody pairs, requiring only 10-20 μl of antibodies and samples. This enhances the ability to measure multiple cytokines from a single well for inflammation assays where supernatant volume is limited.
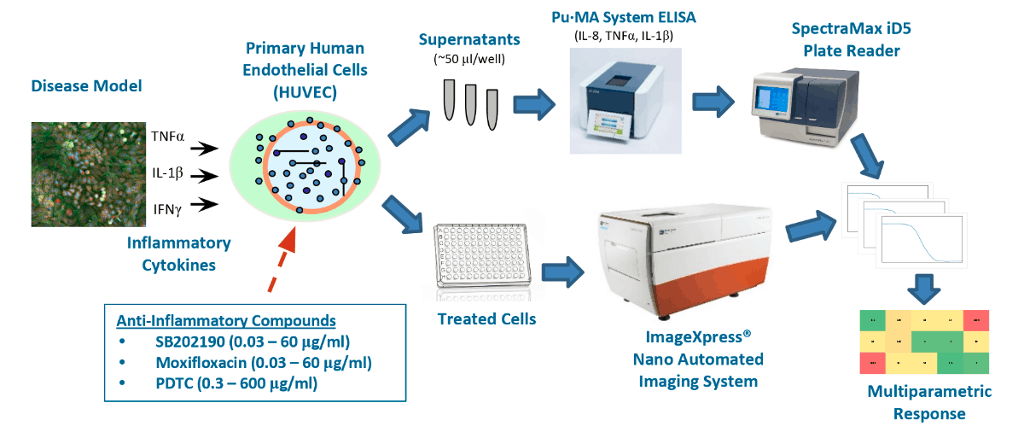
Figure 1. Cartoon of Multiparametric Inflammation assay workflow.
Inflammation Marker Upregulation
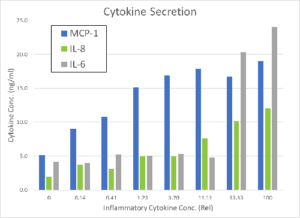
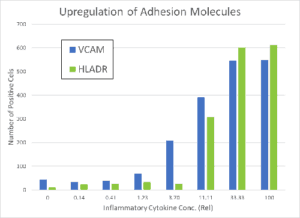
HUVECs were incubated for 48 hrs and then then exposed for 24 hrs to increasing concentrations of the proinflammatory factors IFN-γ, TNF-α, and IL-1β. The Pu·MA System low-volume ELISA was used to quantify the amount of secreted cytokines in cell supernatants, while the ImageXpress Nano automated imaging system was used to quantify cells positive for surface markers. The cytokines MCP-1, IL-8, and IL-6 as well as adhesion molecules VCAM and HLADR were all found to be upregulated in a concentration dependent manner (see Figure 2). A inflammatory cytokine mixture of 5 ng/mL of TNFα, 1 ng/mL IL-1β, and 50 ng/mL of IFNγ was used for subsequent studies of anti-inflammatory drugs.
Protocol
The protocol for the multiparametric inflammation assay is given below. Marker expression results from stimulation with cytokine (CK) mix are shown in Figure 2.
- HUVEC were plated 5,000 cells per well (96-well plate) and incubated for 48 hr. Next they were stimulated with a mix of inflammatory cytokines for 24 hr (5 ng/mL of TNFα, 1 ng/mL IL-1β, 50 ng/mL of IFNγ; all from R&D Systems).
- Anti-inflammatory compounds were added 1 hr prior to CK stimulation.
- After incubation, 60 μl of supernatant was taken for ELISA analysis from each well. The samples were analyzed fresh or stored at -70C for subsequent analysis.
- Cells were then fixed with 4% formaldehyde and stained with antibodies (Ab) for 1 hr and imaged using an ImageXpress Nano Automated Imaging System.
- Ab’s: FITC-mouse anti-human VCAM; PE-mouse anti-human HLA-DR
- Supernatants were diluted 3:1 in assay buffer and analyzed for MCP-1, IL-6 and IL-8 using Pu·MA System flowchip reagents
- All Ab pairs from BioLegend
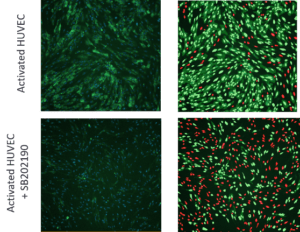
Figure 3. Images of activated HUVEC cells stimulated with CK mix with or without treatment of 10μM SB202190. Left: Cells stained with anti-VCAM antibodies (green) and Hoechst (blue). Right: Analysis masks for nuclei of VCAM-positive cells (light green), cytoplasm of VCAM positive cells (dark green), nuclei of VCAM negative cells (red).
Anti-Inflammatory Drugs
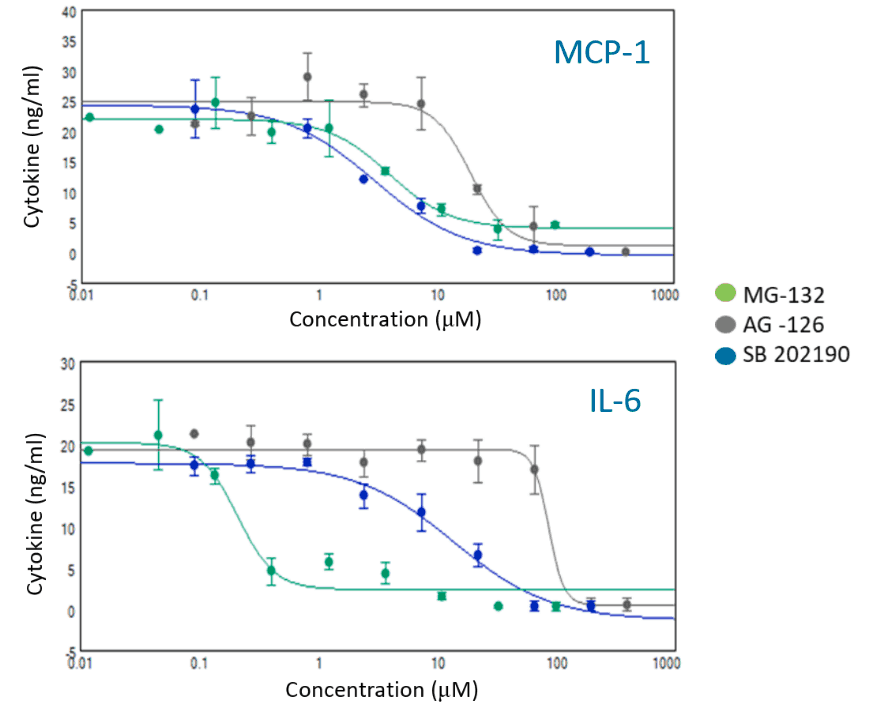
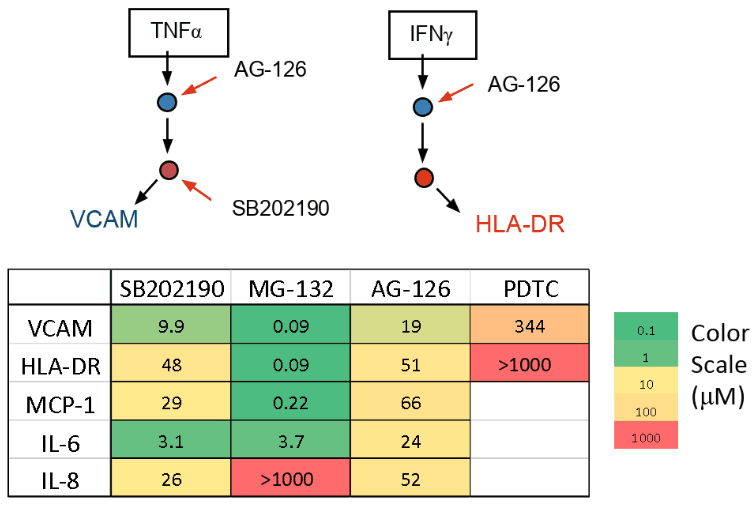
Inflammation is triggered by activation of receptors with cytokines leading to a cascade of signaling events. Kinases activate transcription factors that up-regulate adhesion molecules and cytokines (markers). Different markers are under control of different pathways and transcription factors. We investigated four known compounds that effect different parts of the inflammation pathways and measured the response of five markers.
- MG-132 a proteasome inhibitor, suppresses MCP-1 & IL-6 but stimulates IL-83
- SB202190 a p38 MAPK inhibitor, acts on JAK/STAT and NFκB pathways4
- PDTC an anti-oxidant, suppresses activation of NFκB5
- AG-126 a tyrosine kinase inhibitor, suppresses cytokine secretion and adhesion molecule up-regulation6
Conclusion
- We have demonstrated a multiparametric inflammation assay using a HUVEC cell model with automated cellular imaging and a novel microfluidic ELISA system.
- The Pu·MA System performs immunoassays with existing ELISA antibody pairs using microfluidic flowchips that reduce reagent use and improve time-to-results
- The responses of five different inflammation markers to four anti-inflammatory compounds were characterized. Observed differences in behavior were consistent with published mechanisms of action.
References:
1Proinflammatory Activation Pattern of (HUVEC) cells…; Mako et al, Cytometry Part A, 2010, 77A, 962.
2Method for analyzing signaling networks in complex cellular systems; Plavec I, Sirenko O, et al, PNAS 2004;101(5):1223-8.
3Proteasome inhibition leads to NF- κB-independent IL-8 transactivation…; Hipp et al, Eur J Immunol. 2002, 32, 2208.
4p38α MAP kinase serves cell type-specific inflammatory functions…; Kim et al, Nat Immunol. 2008, 9, 1019.
5PDTC is a potent antioxidant…; Zhu et al, FEBS Letters 2002, 532, 80.
6Cytokine responses of human intestinal epithelial-like Caco-2 cells…; Hosoi et al, Int J Food Microbiol, 2003, 82, 255.
